Senolytics Research Online
Resources and links to further information about senolytics.
https://www.sciencedaily.com/search/?keyword=senolytics#gsc.tab=0&gsc.q=senolytics&gsc.page=1
https://duckduckgo.com/?q=site%3Afightaging.org+Senolytics&ia=web
https://www.sciencedirect.com/search?authors=&cid=&issue=&page=&pub=&qs=Senolytics&show=25&sortBy=relevance&volume=
https://www.ncbi.nlm.nih.gov/pmc/?term=Senolytics
https://pubmed.ncbi.nlm.nih.gov/29988130/
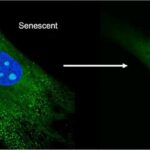
There is a growing body of evidence to support the efficacy of senolytic activators in humans. A number of clinical trials are currently underway to investigate the potential of these drugs to treat age-related diseases such as frailty, osteoarthritis, and type II diabetes. One study published in the journal Nature Medicine in 2018 found that a senolytic cocktail of the drugs dasatinib and quercetin was effective in reducing the number of senescent cells in elderly patients with osteoarthritis. This study showed that the treatment was associated with improved physical function...